Ageless Minds: Exploring Metabolomics in Cognitive Health and Dementia
Metabolic insight Feb 20, 2024

Unsurprisingly, the main risk factor for cognitive decline is age. Luckily, we are not pre-ordained to cognitive decline as we get older: there are also other factors involved, some of which we can influence. In this blog post, we share our insights on cognitive health and metabolomics by comparing previous research with Nightingale Health’s internal findings and two new pre-printed studies.
Different groups of biomarkers in our blood, including some lipids, amino acids, fatty acids and inflammation markers are associated with general cognitive health or in more serious cases, the risk of developing dementia or Alzheimer’s disease1–6. Some of these biomarkers could help with early diagnosis, but more importantly, some may help to identify individuals at high risk far enough in advance that preventative measures are still possible. Fortunately, the metabolomic profiles for different neurodegenerative and cognitive diseases seem to overlap1 , indicating that lifestyle changes could lead to reductions in disease risks across the board.
To showcase the ability of metabolomic biomarkers in predicting future disease risk, Nightingale Health’s internal science team has previously pre-printed findings using metabolomic risk scores for diseases such as type 2 diabetes, cardiovascular disease and COPD. Among these diseases were also Alzheimer’s disease and other dementias, for which we were able to predict future risks independent from age 7,8.
The multi-purpose MetaboHealth score
Not only Nightingale Health’s internal science team, but also a team led by researchers from the Leiden University Medical Center (LUMC) has developed such risk scores. The all-cause mortality score LUMC researchers developed⁹ includes fourteen different serum metabolomic biomarkers involved in inflammation, lipoprotein and fatty acid metabolism, glycolysis, and fluid balance. This score, later coined the MetaboHealth score, was recently used in a pre-print exploring its link with cognitive function in older adults10.
The study involved participants from the Prospective Study of Pravastatin in the Elderly (PROSPER), which focuses on older adults at risk of cardiovascular disease. The researchers examined the association between the MetaboHealth score and cognitive performance. In the study, a higher MetaboHealth score, which indicated increased metabolic disturbance, was linked to poorer cognitive function, and increased functional decline.
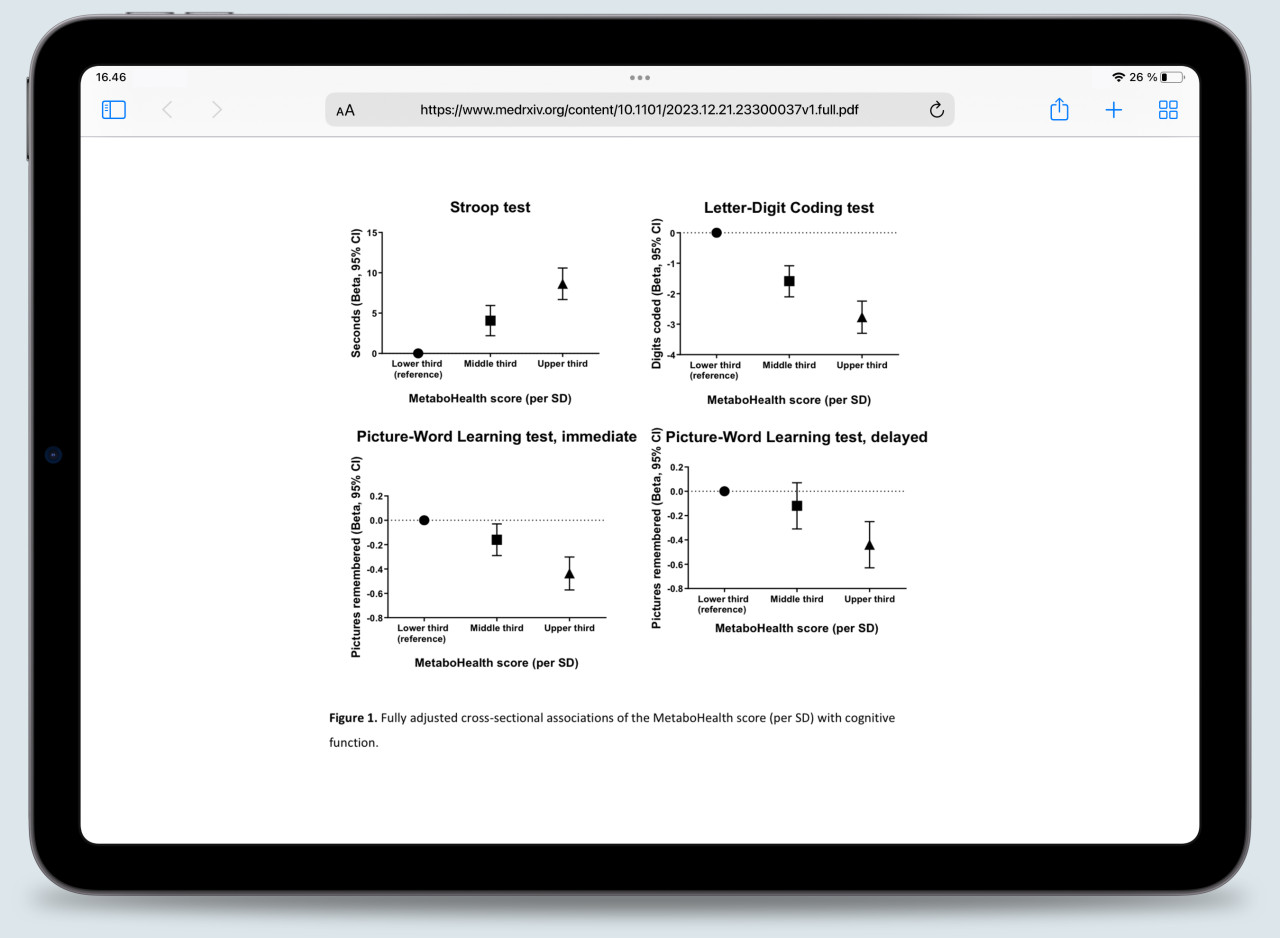
Figure 1. The associations between the MetaboHealth score per standard deviation and cognitive tests, as pre-printed by Zonneveld and colleagues¹⁰.
As the researchers showed in Figure 1, individuals with high MetaboHealth scores consistently performed worse on cognitive tests than individuals with low scores. During follow-up, these individuals also seemed to decline faster than their counterparts.
In the spotlight: inflammatory biomarker GlycA
Researchers behind a second recent pre-print went on a slightly different path and assessed the specific relationship between Alzheimer’s disease and glycoprotein acetyls (GlycA)11. The researchers studied levels of GlycA, a stable biomarker of inflammation, and changes in cognition and brain structures in over 1,500 study participants. The research highlighted that higher levels of GlycA were associated with Alzheimer’s disease, and in patients with mild cognitive impairment at baseline, with a decline in executive functions and memory at follow-up.
On the road to ageless minds
Combining these findings, it's evident that metabolomic biomarkers can partially predict changes in cognitive health and disease. These two most recent examples, the MetaboHealth score and elevated GlycA levels, can not only mark the presence of cognitive impairment or Alzheimer’s disease, but can also predict further deterioration down the line. These insights underscore the importance of monitoring blood biomarkers for early detection and intervention in cognitive decline and dementia. Our minds might not be ageless quite yet, but we’re on the road to taking control.
References
1. Liu, J. et al. Profiling the metabolome of patients with dementia in the UK Biobank. Alzheimer’s & Dementia 17, e056147 (2021).
2. Zhang, X. et al. Plasma metabolomic profiles of dementia: a prospective study of 110,655 participants in the UK Biobank. BMC Medicine 20, 252 (2022).
3. Huang, S.-Y. et al. Investigating Causal Relations Between Circulating Metabolites and Alzheimer’s Disease: A Mendelian Randomization Study. Journal of Alzheimer’s Disease 87, 463–477 (2022).
4. Tynkkynen, J. et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: A prospective study in eight cohorts. Alzheimer’s & Dementia 14, 723–733 (2018).
5. van der Lee, S. J. et al. Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimer’s & Dementia 14, 707–722 (2018).
6. He, Y. et al. Circulating polyunsaturated fatty acids, fish oil supplementation, and risk of incident dementia: a prospective cohort study of 440,750 participants. GeroScience 45, 1997–2009 (2023).
7. Machado-Fragua, M. D. et al. Circulating serum metabolites as predictors of dementia: a machine learning approach in a 21-year follow-up of the Whitehall II cohort study. BMC Medicine 20, 334 (2022).
8. Nightingale Health Biobank Collaborative Group. Metabolomic and genomic prediction of common diseases in 477,706 participants in three national biobanks. 2023.06.09.23291213 Preprint at https://doi.org/10.1101/2023.0... (2023).
9. Deelen, J. et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat Commun 10, 3346 (2019).
10. Zonneveld, M. H. et al. Increased 1H-NMR metabolomics-based health score associates with declined cognitive performance and functional independence in older adults at risk of cardiovascular disease. 2023.12.21.23300037 Preprint at https://doi.org/10.1101/2023.1... (2023).
11. Liang, N. et al. Peripheral inflammation is associated with structural brain atrophy and cognitive decline linked to mild cognitive impairment and Alzheimer’s disease. 2023.12.08.23299734 Preprint at https://doi.org/10.1101/2023.1... (2023).
